Bilirubin, a beneficial antioxidant at low levels,can progress to neurotoxic properties at higher levels where it can impair the normal developmental maturation of the neonatal brain. As a “silent toxin”, deleterious effects are exacerbated by co-morbidities such as inflammation/sepsis, oxidative stress, prematurity, and even sociocultural environmental factors. Failure to recognize or identify infants at high risk for developing extreme hyperbilirubinemia can lead to severe neurologic sequelae, which include athetoid cerebral palsy (Kernicterus) to a range of subtle cellular damage of bilirubin induced neurological dysfunction (BIND) that appear to be gestational age-related. The clinical dilemma is that the sensitivity and specificity of current measures of total bilirubin (TB) levels to discriminate the precise thresholds between safe and worrisome TB levels are not adequate. In fact, often in infants <28 wks gestational age, there is a no specific peak TB level that is clearly associated with neurologic impairments.
Pre-clinical studies have illustrated the development of neurogenic niches and of endothelial cells, together with microglia migration, that are significantly vulnerable to bilirubin neurotoxicity. In the opinions of several distinguished authors and experts the risk versus benefit of initiating interventions or withholding interventions for any infant at high risk for developing extreme hyperbilirubinemia currently rely on immaturity of the brain, TB assay and caution.
THUS, THE CURRENT APPROACH TO A NEWBORN AT RISK FOR NEWBORN JAUNDICE IS BASED ON THREE PRINCIPLES:
- system-based screening for risk assessment that include bilirubin (TB), gestational age and hemolysis.
- unfettered access to effective phototherapy.
- surveillance for subtle bilirubin neurotoxicity for those who have reached or exceed the AAP (American Academy of Pediatrics) recommended bilirubin thresholds for intervention.
Effective phototherapy is the use of a specific wavelength and dose of light (photons) delivered to an exposed body surface area for the purpose of reducing the neurotoxic levels of bilirubin. Light needs to be prescribed as a drug. It is usually prophylactic with a goal of containing the rate-ofrise of bilirubin. The most effective phototherapy is administered using blue light in the wavelength range of 469 +/- 10nm. A recent technical report from the AAP details further evidence and operational considerations. Interference light dispersion to reach the bilirubin molecule may modulate the effectiveness of irradiance delivered.
EFFECTIVENESS OF PHOTOTHERAPY IS DEFINED BY:
- Measurements of irradiance at regular intervals with an appropriate spectroradiometer to validate dose for the specific wavelength that is uniformly
distributed over the light footprint. - Adjusting the exposed surface area to increase exposure to light source and thereby increase the “dose”. Such an approach is useful if the TB continues
to rise while the infant is being administered phototherapy. Additional phototherapy devices may be used to widen the exposure of the BSA. - Increasing the irradiance to a higher intensity setting on the device or by bringing the overhead light closer to the infant. Certain Light emitting
diode light sources can be brought closer to an infant, but this cannot be done with halogen or tungsten lamps because of the danger of a burn. - Discontinuation of phototherapy when TB is 1 to 2 mg/dL (17-34 mmol/L) below the suggested initiation level for an infant’s postmenstrual age.
- Discontinuation of TB measurements when TB is lowered in the absence of phototherapy.
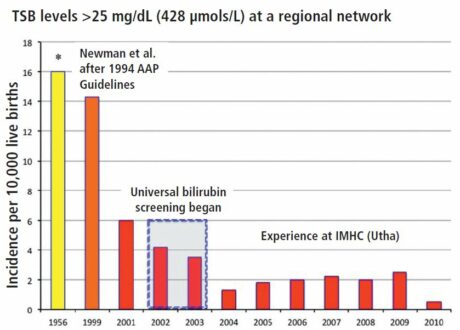
Figure 1. Systems approach implementation and its impact on the incidence of extreme hyperbilirubinemia in a regional network (red bars). (Yellow bar represent data from Collaborarive Perinatal Project; orange bar represents data from Newman et al, 1999)
The most easily preventable neonatal condition
From a regional perspective, implementation of such an approach has a beneficial outcome by reducing extreme hyperbilirubinemia (Figure 1). Among individual newborns, manifestations of bilirubin neurotoxicity may be assessed by clinical signs of BIND, auditory brain stem screening and (if the risk is high) by magnetic resonance imaging. Those at most risk should be followed during infancy and childhood for neuromotor and movement disorders, upward gaze abnormalities, auditory neuropathies and sensory processing disorders. Cognitive impairments are unusual but are more likely for disordered expressive and receptive language development. Overall,a systems approach among high-income communities, regions, or nations have been associated with reduced burden of jaundice-related adverse effects; however, global challenges remain and urgent action is needed to manage the most easily preventable neonatal condition.
VINOD K. BHUTANI, MD, FAAP / Professor of Pediatrics, Division of Neonatal-Perinatal & Developmental Medicine, Stanford Children’s Health, Stanford University School of Medicine. Stanford, CA. 94305
2 responses on "Reduce adverse burden of newborn jaundice"
Leave a Message
You must be logged in to post a comment.




There is no problem with treating infants with Phototherapy whilst being nursed in Incubators – please check the manufacturers recommendation on the Light intensity at certain distance in order to ascertain how far away the lamp should be from the infant in order to achieve the desired intensity. You can check this intensity additionally at the bed surface by using the radiometer recommended by the manufacturer of the phototherapy lamp.
Most full term infants are able to adequately maintain temperature in bassinets, so long as the environmental temperature is adequate for warmth – Keeping babies warm in open bassinet style cots can alternatively be achieved either by using a Perspex shield over the top of the bassinet to prevent convective heat exchange, or alternatively by placing a warming pad beneath the infant. It is important to regularly check the baby temperature independently, and in the case that the infant is unable to maintain temperature, it would be recommended to nurse them in an incubator / radiant warmer during the phototherapy treatment, based on their clinical condition.
Is there any concern with treating the newborn in an incubator with regards to the “dose” potentially being minimized as the light has to pass through the wall or walls of the ceiling of the incubator?
Also, any tips on how to keep the newborn warm when treating in an open cot as is pictured above would be greatly appreciated.
Thanks so much.
Shelley Buckborough RN, Sales